
The enthalpy changes for many types of chemical and physical processes are available in the reference literature, including those for combustion reactions, phase transitions, and formation reactions. As we discuss these quantities, it is important to pay attention to the extensive nature of enthalpy and enthalpy changes. Since the enthalpy change for a given reaction is proportional to the amounts of substances involved, it may be reported on that basis (i.e., as the ΔH for specific amounts of reactants). However, we often find it more useful to divide one extensive property (ΔH) by another (amount of substance), and report a per-amount intensive value of ΔH, often “normalized” to a per-mole basis. (Note that this is similar to determining the intensive property specific heat from the extensive property heat capacity, as seen previously.) A standard state is a commonly accepted set of conditions used as a reference point for the determination of properties under other different conditions. For chemists, the IUPAC standard state refers to materials under a pressure of 1 bar and solutions at 1 M, and does not specify a temperature (it used too). Many thermochemical tables list values with a standard state of 1 atm. Because the ΔH of a reaction changes very little with such small changes in pressure (1 bar = 0.987 atm), ΔH values (except for the most precisely measured values) are essentially the same under both sets of standard conditions. We will include a superscripted “o” in the enthalpy change symbol to designate standard state. Since the usual (but not technically standard) temperature is 298.15 K, we will use a subscripted “298” to designate this temperature. Thus, the symbol (\(ΔH^\circ_\)) is used to indicate an enthalpy change for a process occurring under these conditions. (The symbol ΔH is used to indicate an enthalpy change for a reaction occurring under nonstandard conditions.)
Standard enthalpy of combustio n (\(ΔH_C^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under standard state conditions; it is sometimes called “heat of combustion.” For example, the enthalpy of combustion of ethanol, −1366.8 kJ/mol, is the amount of heat produced when one mole of ethanol undergoes complete combustion at 25 °C and 1 atmosphere pressure, yielding products also at 25 °C and 1 atm. \[\ce
| Substance | Combustion Reaction | Enthalpy of Combustion \(ΔH_c^\circ \left(\mathrm \:at\:25°C>\right)\) |
|---|---|---|
| carbon | \(\ce(s)+\ce(g)⟶\ce(g)\) | −393.5 |
| hydrogen | \(\ce(g)+\frac\ce(g)⟶\ce(l)\) | −285.8 |
| magnesium | \(\ce(s)+\frac\ce(g)⟶\ce(s)\) | −601.6 |
| sulfur | \(\ce(s)+\ce(g)⟶\ce(g)\) | −296.8 |
| carbon monoxide | \(\ce(g)+\frac\ce(g)⟶\ce(g)\) | −283.0 |
| methane | \(\ce(g)+\ce(g)⟶\ce(g)+\ce(l)\) | −890.8 |
| acetylene | \(\ce(g)+\dfrac\ce(g)⟶\ce(g)+\ce(l)\) | −1301.1 |
| ethanol | \(\ce(l)+\ce(g)⟶\ce(g)+\ce(l)\) | −1366.8 |
| methanol | \(\ce(l)+\dfrac\ce(g)⟶\ce(g)+\ce(l)\) | −726.1 |
| isooctane | \(\ce(l)+\dfrac\ce(g)⟶\ce(g)+\ce(l)\) | −5461 |

Example \(\PageIndex<3>\): Using Enthalpy of Combustion As Figure \(\PageIndex<3>\) suggests, the combustion of gasoline is a highly exothermic process. Let us determine the approximate amount of heat produced by burning 1.00 L of gasoline, assuming the enthalpy of combustion of gasoline is the same as that of isooctane, a common component of gasoline. The density of isooctane is 0.692 g/mL. Figure \(\PageIndex<3>\): The combustion of gasoline is very exothermic. (credit: modification of work by “AlexEagle”/Flickr) Solution Starting with a known amount (1.00 L of isooctane), we can perform conversions between units until we arrive at the desired amount of heat or energy. The enthalpy of combustion of isooctane provides one of the necessary conversions. Table \(\PageIndex\) gives this value as −5460 kJ per 1 mole of isooctane (C8H18). Using these data,
\[\mathrm>×\dfrac>>>>×\dfrac>>>>×\dfrac>>>>×\dfrac>>=−3.31×10^4\:kJ> \nonumber\]The combustion of 1.00 L of isooctane produces 33,100 kJ of heat. (This amount of energy is enough to melt 99.2 kg, or about 218 lbs, of ice.) Note: If you do this calculation one step at a time, you would find: \(\beginExercise \(\PageIndex<3>\) How much heat is produced by the combustion of 125 g of acetylene? Answer 6.25 × 10 3 kJ
Emerging Algae-Based Energy Technologies (Biofuels) As reserves of fossil fuels diminish and become more costly to extract, the search is ongoing for replacement fuel sources for the future. Among the most promising biofuels are those derived from algae (Figure \(\PageIndex<4>\)). The species of algae used are nontoxic, biodegradable, and among the world’s fastest growing organisms. About 50% of algal weight is oil, which can be readily converted into fuel such as biodiesel. Algae can yield 26,000 gallons of biofuel per hectare—much more energy per acre than other crops. Some strains of algae can flourish in brackish water that is not usable for growing other crops. Algae can produce biodiesel, biogasoline, ethanol, butanol, methane, and even jet fuel.  According to the US Department of Energy, only 39,000 square kilometers (about 0.4% of the land mass of the US or less than \(\dfrac\) of the area used to grow corn) can produce enough algal fuel to replace all the petroleum-based fuel used in the US. The cost of algal fuels is becoming more competitive—for instance, the US Air Force is producing jet fuel from algae at a total cost of under $5 per gallon. The process used to produce algal fuel is as follows: grow the algae (which use sunlight as their energy source and CO2 as a raw material); harvest the algae; extract the fuel compounds (or precursor compounds); process as necessary (e.g., perform a transesterification reaction to make biodiesel); purify; and distribute (Figure \(\PageIndex\)).
According to the US Department of Energy, only 39,000 square kilometers (about 0.4% of the land mass of the US or less than \(\dfrac\) of the area used to grow corn) can produce enough algal fuel to replace all the petroleum-based fuel used in the US. The cost of algal fuels is becoming more competitive—for instance, the US Air Force is producing jet fuel from algae at a total cost of under $5 per gallon. The process used to produce algal fuel is as follows: grow the algae (which use sunlight as their energy source and CO2 as a raw material); harvest the algae; extract the fuel compounds (or precursor compounds); process as necessary (e.g., perform a transesterification reaction to make biodiesel); purify; and distribute (Figure \(\PageIndex\)). 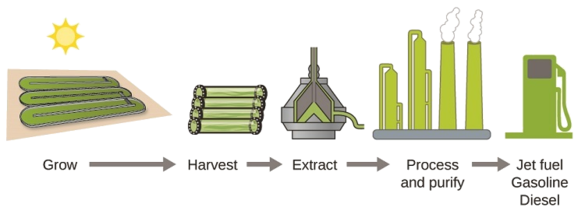
A standard enthalpy of formation \(ΔH^\circ_\ce
Example \(\PageIndex<4>\): Evaluating an Enthalpy of Formation Ozone, O3(g), forms from oxygen, O2(g), by an endothermic process. Ultraviolet radiation is the source of the energy that drives this reaction in the upper atmosphere. Assuming that both the reactants and products of the reaction are in their standard states, determine the standard enthalpy of formation, \(ΔH^\circ_\ce\) of ozone from the following information:
\[\ce<3O2>(g)⟶\ce(g)\hspaceΔH^\circ_=+286\: \ce \nonumber\]S olutio n \(ΔH^\circ_\ce
Solution
Remembering that \(ΔH^\circ_\ce\) reaction equations are for forming 1 mole of the compound from its constituent elements under standard conditions, we have:
Note: The standard state of carbon is graphite, and phosphorus exists as \(P_4\).
Write the heat of formation reaction equations for: